Hydrogen–deuterium exchange mass spectrometry
For higher order structure studies, hydrogen deuterium exchange with mass spectrometry – alternatively referred to as H/D exchange, HDX, or HXMS – has become indispensable for mapping protein-protein and protein-ligand domains. Recent innovations in LC/MS, automation, and informatics technologies have converted HDX MS from a complex academic exercise to a robust tool for discovery and development of protein drugs. This system leverages high-pressure nano- to microscale UPLC separations and high-resolution MSE mass spectrometry to answer important questions about biotherapeutic protein dynamics, conformation, and interactions, including:
- Epitope mapping
- Protein-protein interactions
- Protein-drug binding
- Localization of conformational changes as a result of formulations and stability testing
- Aggregation
- Effects of mutation on protein conformation and dynamics
Principles of HDX
Hydrogen deuterium exchange (HDX) MS works in many applications, including understanding how small-molecule therapeutics bind to protein targets, and performing epitope mapping. With this technology, deuterium in a solution replaces the amide hydrogen in a biomolecule’s backbone, and MS measures these changes. The results reveal information about protein structure.
Basic principles of hydrogen deuterium exchange
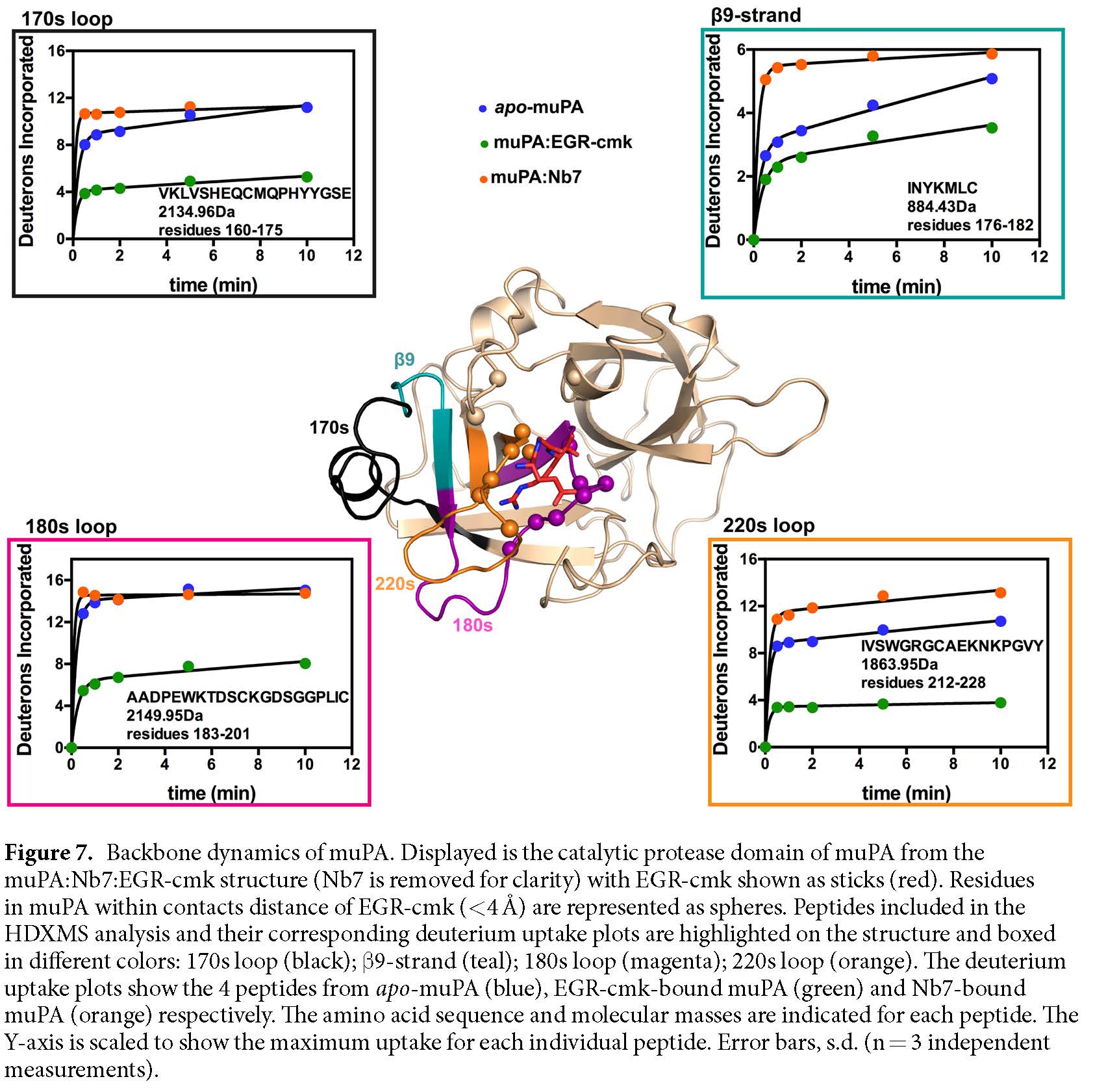
Amide hydrogen on the backbone of a biomolecule exchanges with deuterium in solution at different rates partly dependent on conformation. These changes can be measured by MS and therefore the degree of activity at various sites can be inferred. These locations can be identified and mapped to the primary sequence, which also allows easier visualization. Relative folding and dynamics can be determined from the different rates of uptake at different locations (see example below from Kromann-Hansen et al., 2017).
Recent publications using the BPMSF Synapt G2Si:
- Discovery of a novel conformational equilibrium in urokinase-type plasminogen activator; Kromann-Hansen et al., Nature 2017
- Monomeric ephrinB2 binding induces allosteric changes in Nipah virus G that precede its full activation; Wong et al., Nature Communications 2017
For more detail on the Synapt G2Si Instrument:
For NEW HDX projects please directly contact Dr. Komives or Dr. Silletti.
If you already have discussed your project with Dr. Komives or Dr. Silletti, please fill out
and please sign-up on the Synapt calendar
For more information about Elizabeth Komives' research you can visit the Komives Lab website